Ottawa – The latest edition of the Patented Medicine Prices Review Board (PMPRB) Meds Pipeline Monitor reports that oncology continued to dominate the therapeutic mix of the drug pipeline in 2022, with cancer treatments representing nearly one third (30%) of medicines in all phases of clinical trials. Treatments for infectious diseases held the second largest share of the pipeline, at 15%, due to the increased number of medicines for the treatment of COVID-19.
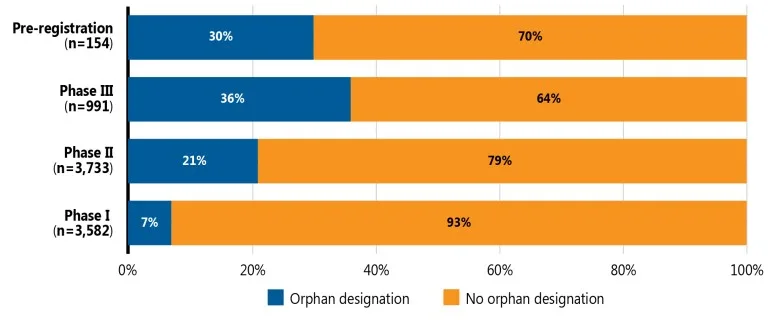
Meds Pipeline Monitor 2022 reviewed 1,257 new medicines in late stages of clinical evaluation to highlight drugs that could potentially impact the Canadian health care system. Among these, the PMPRB identified 28 late-stage new medicines, including five new gene therapies, that may offer breakthroughs in treating previously unmet needs or have the potential to treat large patient populations, as well as five new medicines currently under review by Heath Canada. The report also provides an overview of the fast-moving pipeline for medicines and vaccines under development to treat and prevent COVID-19.
The Meds Pipeline Monitor series, which is published through the National Prescription Drug Utilization Information System (NPDUIS) research initiative, provides a snapshot of the new drug landscape, highlighting medicines currently in clinical trials that may significantly impact future clinical practice and/or drug spending in Canada if approved for sale.
This annual horizon-scanning report is part of a suite of PMPRB publications that examine key segments of the pharmaceutical market. Together with its companion publication, Meds Entry Watch, it examines the continuum of new and emerging medicines in Canada and internationally.