Summary
Product
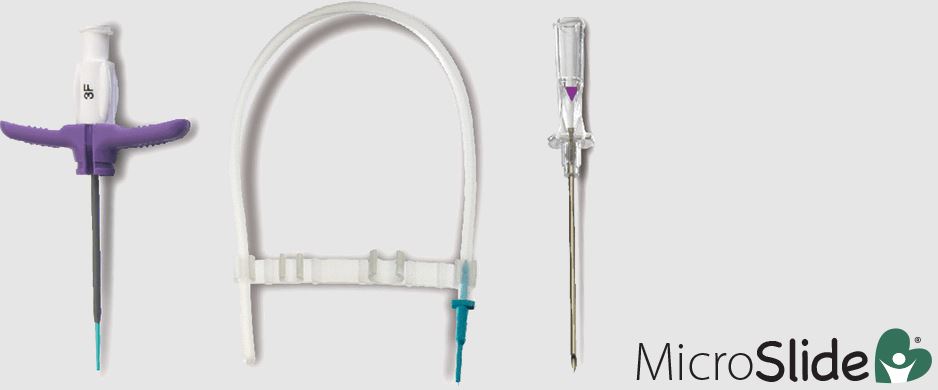
Tearaway Microslide Introducer Kit
Issue
Medical devices – Sterility
What to do
Contact the manufacturer if you require additional information.
Audience
Healthcare
Affected products
Issue
Sterilized finished devices associated with the lots referenced in this recall may have an open seal in the sterile barrier packaging of the device.
Recall start date: March 28, 2025
Additional information
Details
Original published date:
Alert / recall type
Health product recall
Category
Health products – Medical devices – Cardiovascular
Companies
| Galt Medical Corporation |
| 2220 Merritt Drive, Garland, Texas, United States, 75041 |
Published by
Health Canada
Audience
Healthcare
Recall class
Type II
Identification number
RA-77262