MISSISSAUGA — The Ontario government has secured a $820 million investment from AstraZeneca, a global biopharmaceutical business, to expand the company’s research and development (R&D) facilities in Mississauga and relocate the company’s Canadian headquarters to a larger, state-of-the-art office. The investment will create over 700 new, good-paying jobs across Ontario’s life sciences sector and further establish the province as a global …
AstraZeneca’s Calquence improves chronic lymphocytic leukaemia
London – Positive high-level results from an interim analysis of the AMPLIFY Phase III trial showed a fixed duration of AstraZeneca’s Calquence (acalabrutinib) in combination with venetoclax, with or without obinutuzumab, demonstrated a statistically significant and clinically meaningful improvement in progression-free survival (PFS) compared to standard-of-care chemoimmunotherapy in previously untreated adult patients with chronic lymphocytic leukaemia (CLL). For the secondary …
Acquisition of Amolyt Pharma completed
London – AstraZeneca announced the successful completion of the acquisition of Amolyt Pharma, a clinical-stage biotechnology company focused on developing novel treatments for rare endocrine diseases. The acquisition bolsters the Alexion, AstraZeneca Rare Disease late-stage pipeline and expands on its bone metabolism franchise with the notable addition of eneboparatide (AZP-3601), a Phase III investigational therapeutic peptide with a novel mechanism of …
Tagrisso with addition of chemotherapy approved in EU
London – AstraZeneca’s Tagrisso (osimertinib) with the addition of pemetrexed and platinum-based chemotherapy has been approved in the European Union (EU) for the 1st-line treatment of adult patients with advanced epidermal growth factor receptor-mutated (EGFRm) non-small cell lung cancer (NSCLC) whose tumours have exon 19 deletions or exon 21 (L858R) mutations. The approval by the European Commission follows the positive opinion of the Committee …
Tagrisso reduced risk of progression in mutated lung cancer
London – Positive results from the LAURA Phase III trial showed AstraZeneca’s Tagrisso (osimertinib) demonstrated a statistically significant and highly clinically meaningful improvement in progression-free survival (PFS) for patients with unresectable, Stage III epidermal growth factor receptor-mutated (EGFRm) non-small cell lung cancer (NSCLC) whose tumours have exon 19 deletions or exon 21 (L858R) mutations, after chemoradiotherapy (CRT) compared to placebo after CRT. …
AstraZeneca redefining lung, metastatic breast cancer treatment
Washington – AstraZeneca advances its ambition to redefine cancer care with new data across its industry-leading portfolio and pipeline at the American Society of Clinical Oncology (ASCO) Annual Meeting, 31 May to 4 June 2024. More than 100 abstracts will feature 25 approved and potential new medicines across the Company’s diverse oncology portfolio and pipeline, including two late-breaking plenary presentations, …
AstraZeneca to set up antibody drug facility in Singapore
London – AstraZeneca intends to build a $1.5 billion manufacturing facility in Singapore for antibody drug conjugates (ADCs), enhancing global supply of its ADC portfolio. ADCs are next-generation treatments that deliver highly potent cancer-killing agents directly to cancer cells through a targeted antibody. The planned greenfield facility, supported by the Singapore Economic Development Board (EDB), will be AstraZeneca’s first end-to-end …
AstraZeneca withdraws Covid-19 vaccine
London – According to The Telegraph, amid “rare and dangerous side effect” of its COVID-19 vaccine, UK-based pharmaceutical manufacturer AstraZeneca has announced withdrawal of its inoculation jab. It will also be withdraw Vaxzevria’s marketing authorisations within Europe. The firm’s application to withdraw the vaccine was made on March 5 and came into effect on May 7.
Imfinzi plus chemotherapy doubled overall survival rate
London – Updated exploratory results from the TOPAZ-1 Phase III trial showed AstraZeneca’s Imfinzi (durvalumab) in combination with standard-of-care chemotherapy demonstrated a clinically meaningful long-term overall survival (OS) benefit at three years for patients with advanced biliary tract cancer (BTC). These results from TOPAZ-1, which are the longest survival follow-up ever reported for a global, randomised Phase III trial in this …
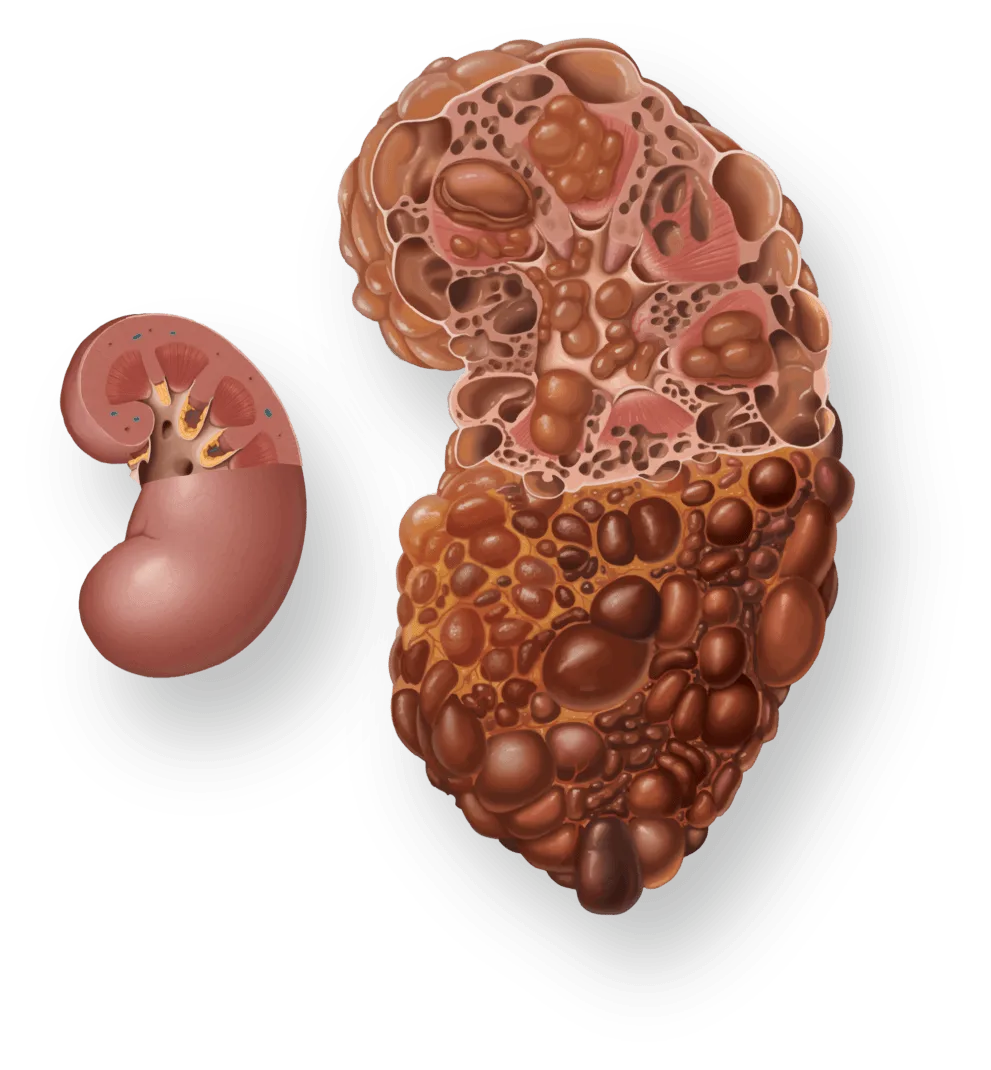
New modelling reveals escalating toll of chronic kidney disease
London – A new modelling analysis by AstraZeneca, IMPACT CKD, forecasts up to 16.5% of the population across eight countries will suffer from chronic kidney disease (CKD) by 2032, including a rise of up to 59.3% in advanced-stage.1 Presented at the 2024 ISN World Congress of Nephrology (WCN’24) in Buenos Aires, the study highlights an urgent and growing global health crisis …