Ottawa – Health Canada has authorized the use of the Moderna SPIKEVAX™ Covid-19 vaccine targeting the Omicron XBB.1.5 subvariant for people six months of age and older. Health Canada received Moderna’s submission for its new Covid-19 vaccine on June 29, 2023. After a thorough, independent review of the evidence, Health Canada has determined that the vaccine meets the Department’s stringent safety, …
Ministers of Health talk about Fetal Alcohol Spectrum Disorder
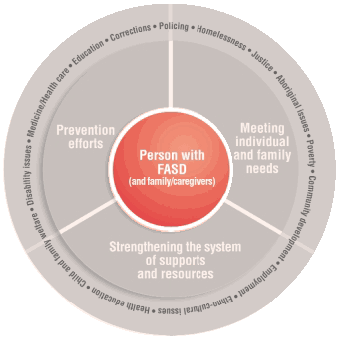
Ottawa – Each year we mark Fetal Alcohol Spectrum Disorder (FASD) Awareness Day and FASD Awareness Month to raise awareness about the risks of drinking alcohol during pregnancy, highlight prevention and support efforts, and encourage individuals to learn more about FASD and its impacts. Early intervention services help. The theme for this year’s FASD Awareness Month is Uniting Our Strengths: Finding …
Weight loss drug can help treating diabetes
London – Researchers found in their new study, published in the New England Journal of Medicine, that Ozempic or Wegovy, drug used for weight loss — semaglutide — might contribute to reducing or completely cutting the insulin shots in people diagnosed with Type 1 diabetes. They analysed information from 10 people with Type 1 diabetes who took the drug. who …
Moderna: Updated Covid vax is effective against BA.2.86 variant
Washington – Moderna said its clinical trial data showed its updated Covid-19 vaccine can effective against the highly-mutated BA.2.86 subvariant that has raised fears of a resurgence of infections. Moderna head of infectious diseases Jacqueline Miller said in an interview: “We think this is news people will want to hear as they prepare to go out and get their fall …
Calquence approved in China for chronic lymphocytic leukaemia
London – AstraZeneca’s Calquence (acalabrutinib), a next generation, selective Bruton’s tyrosine kinase (BTK) inhibitor, has been approved in China for the treatment of adult patients with chronic lymphocytic leukaemia (CLL) or small lymphocytic lymphoma (SLL) who have received at least one prior therapy. The approval by the National Medical Products Administration (NMPA) was based on positive results from two clinical trials, including …
Highly mutated Coronavirus variant detected in Canada, what’s cure
Ottawa – Health officials in Canada has identified BA.2.86 variant of the Omicron coronavirus in the country – found in an individual in British Columbia who had not travelled outside the region. He has not currently in hospital, and the discovery does not alter the level of risk to the people in British Columbia. Canadian health authorities says overall virus …
Narcan to be available as over-the-counter drug
Washington – The overdose reversal drug Narcan manufacturer announced the Food and Drug Administration (FDA) approved nasal spray and it will be available for over-the-counter purchase starting in September. According to Biotech company Emergent BioSolutions, the Narcan nasal spray has already been shipped to pharmacies, drugstores, grocery stores and online retailers.
Botswana National HIV Reference Lab designated WHO Collaborating Centre
Gaborone – The Republic of Botswana and the World Health Organization announced designation of the Botswana National HIV Reference Laboratory as a WHO Collaborating Centre, which will enable deeper collaboration with WHO in advancing the health and well-being of people living with HIV. Botswana has made tremendous strides in improving its diagnostic and laboratory systems, particularly in the areas of …
Toronto proclaims Overdose Awareness Day: Discuss cure, medications
Toronto – The City of Toronto has proclaimed today, August 31, Overdose Awareness Day, aligned with the City’s participation in International Overdose Awareness Day (IOAD), the world’s largest annual initiative to raise awareness of and end drug overdoses. IOAD serves to unite communities worldwide in a shared commitment to end all preventable overdose-related deaths. The day is dedicated to fostering …
FDA warns against 2 brands of eyedrops
Washington – The Food and Drug Administration (FDA) issued a warning to consumers not to use certain kinds of eyedrops as they may include unapproved medications that cannot be sold legally in the US and may be infected with bacteria, fungus, or both. Consumers were advised not to use Dr Berne’s MSM Drops 5% Solution and LightEyez MSM Eye Drops. …