Charlottetown – No Canadian should have to choose between paying for prescription drugs and putting food on the table. Unfortunately, many are faced with this impossible decision every day. The Government of Canada recognizes that for many Canadians, the cost of their medication is an additional barrier to accessing the essential health care they need. Mark Holland, Canada’s Minister of …
Forever chemicals found in period products. What’s cure?
Washington – Scientists have found presence of toxic forever chemicals — per and polyfluoroalkyl substances (PFAS) — in products used by females during menstruation, which, researchers think, might have been deliberately added, per an Independent report. Researchers said these products are designed to make people feel comfortable during their periods, their labels do not usually contain a list of the ingredients. …
New Appointment at Patented Medicine Prices Review Board
Ottawa – Mark Holland, Minister of Health, announced a new appointment to the Patented Medicine Prices Review Board (PMPRB). Following a merit-based selection process, Anie Perrault is appointed as Vice Chairperson for a five-year term. With over 30 years of experience in the public and private sectors, Ms. Perrault held several positions in the genomic research and biotechnology sector both in Quebec …
FDA approves first postnatal depression pill
Washington – The Food and Drug Administration (FDA) has given approval for the zuranolone, sold under the brand name Zurzuvae, to be used as a pill to treat postnatal depression as alternative to intravenous injection. The drug can be taken by patients as a once-daily pill for two weeks and, per drug manufacturers Sage Therapeutics and Biogen’s direction. The pill …
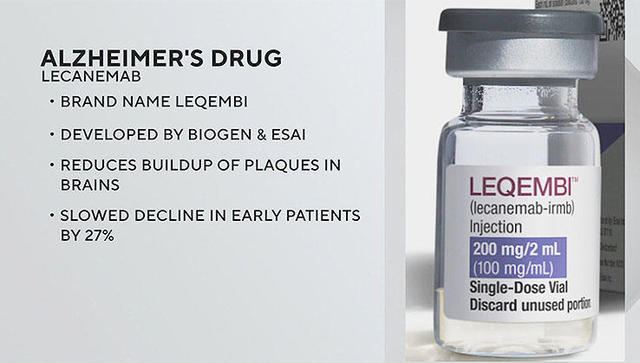
Lilly’s Landmark Phase 3 Trial of Donanemab Presented at Alzheimer’s Association Conference
TORONTO – Eli Lilly and Company presented full results from the Phase 3 TRAILBLAZER-ALZ 2 study showing that donanemab significantly slowed cognitive and functional decline in people with early symptomatic Alzheimer’s disease (AD). The data were shared at the 2023 Alzheimer’s Association International Conference (AAIC) as a featured symposium and simultaneously published in the Journal of the American Medical Association (JAMA). …
AstraZeneca Vaxzevria COVID-19 vaccine
Ottawa – All COVID-19 vaccines authorized in Canada are proven safe, effective and of high quality. Name: AstraZeneca Vaxzevria® COVID-19 vaccine Manufacturer: AstraZeneca Canada Inc and Verity/SII (COVISHIELD) Type: Viral vector-based Status: Approved by Health Canada Approved for: Age 18 and older How it’s given: Injection in muscle (usually the upper arm) Number of doses: 2 doses Note: The COVISHIELD version of this vaccine provided a temporary …
MODERNA AND NOVOCOL PHARMA ANNOUNCE FILL-FINISH AGREEMENT FOR CANADIAN-MADE MRNA VACCINES
Vaccines manufactured at Moderna’s state-of-the-art Canadian mRNA facility to be prepared and packaged at Novocol’s Ontario-based facility Fill-finish agreement will secure domestic end-to-end vaccine production and ensures rapid response capabilities for future pandemic readiness for Canadians TORONTO-Moderna, Inc. (NASDAQ:MRNA), a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, today announced a long-term agreement with Ontario-based Novocol Pharma, a leading …
MODERNA INITIATES ROLLING SUBMISSION TO HEALTH CANADA FOR UPDATED COVID-19 VACCINE
Health Canada submission follows international recommendations to develop monovalent XBB.1.5 COVID-19 vaccines Moderna is prepared to deliver updated COVID-19 vaccines in time for the fall 2023 vaccination season TORONTO – Moderna, Inc. (NASDAQ:MRNA), a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, announced that it has initiated the filing of a rolling New Drug Submission (NDS) to Health Canada …
Tagrisso shows survival in early-stage EGFR-mutated lung cancer
London – Positive results from the ADAURA Phase III trial showed AstraZeneca’s Tagrisso (osimertinib) demonstrated a statistically significant and clinically meaningful improvement in overall survival (OS), compared to placebo in the adjuvant treatment of patients with early-stage (IB, II and IIIA) epidermal growth factor receptor-mutated (EGFRm) non-small cell lung cancer (NSCLC) after complete tumour resection with curative intent. These results will be …
FDA approves Biogen-Sage Therapeutics pill for postpartum depression
Washington – The US Food and Drug Administration (FDA) approved Biogen BIIB.O and Sage Therapeutics’ SAGE.O oral pill to treat postpartum depression (PPD) in adults. The companies had sought the FDA’s approval for the drug, Zurzuvae, to treat major depressive disorderdrug, Zurzuvae, to treat major depressive disorder (MDD), or clinical depression, as well as postpartum depression, which affect millions of …