Product
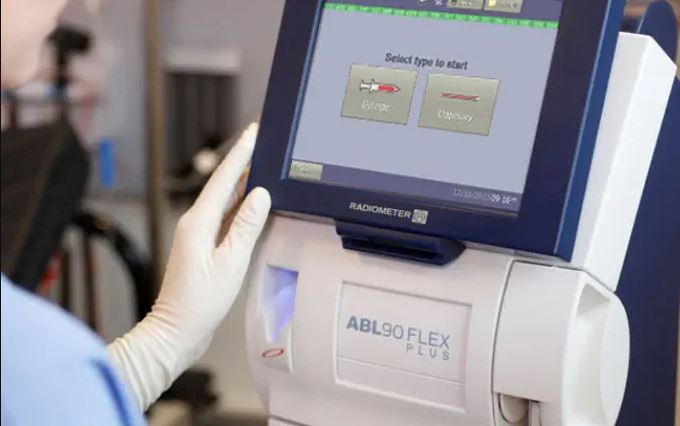
ABL90 Flex and Flex Plus
Issue
Medical devices – Performance
What to do
Contact the manufacturer if you require additional information.
Affected products
Issue
There is a potential risk with ABL90 Flex & ABL90 Flex Plus running RWIN10 1.0 and RWIN10 1.1. The issue is that the analyzer software may unexpectedly freeze during a sample measurement. The analyzer software includes a watchdog that detects a freeze and automatically restarts the software. If the analyzer freezes, a sample being processed will be lost.
Recall Start Date: March 11, 2025
Additional information
Details
Original published date:
Alert / recall type
Health product recall
Category
Health products – Medical devices – Chemistry
Companies
Radiometer Medical Aps
Akandevej 21, Bronshoj, Denmark, 2700
Published by
Health Canada
Audience
Healthcare
Industry
Recall class
Type II
Identification number
RA-77178