Summary
Product
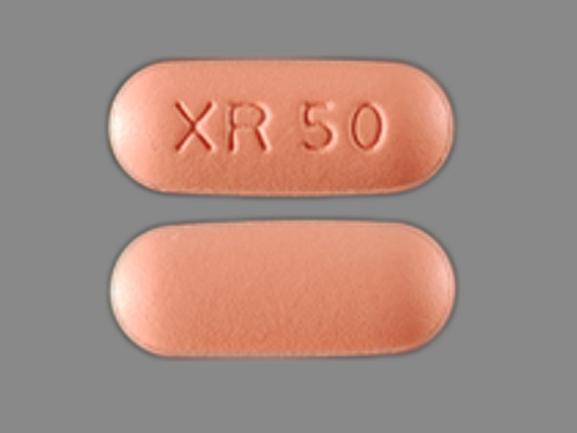
NRA-Quetiapine XR
Issue
Health products – Product quality
What to do
Consult your healthcare provider prior to discontinuing use of the affected product, or for any health concerns.
Distribution
National
Affected products
Filter items
Showing 1 to 1 of 1 entries
Show 102550100 entries
Brand
Product Name
Market Authorization
Dosage Form
Strength
Lot numbers
| NORA PHARMA INC | NRA-Quetiapine XR | DIN 02510677 | Tablet (Extended-Release) | QUETIAPINE FUMARATE 50mg | M2216571 & M2214003 |
Issue
Affected lots may exceed the established acceptable intake limit for N-nitroso-desalkyl-quetiapine (NDAQ)
What you should do
- Verify if your product is affected.
- Consult your healthcare provider prior to discontinuing use of the affected product(s), or for any health concerns.
- Contact the recalling firm if you have any questions about the recall.
- Report any health product related side effects to Health Canada.
- Report any other health product safety complaints to Health Canada.
Additional information
Background
Depth of recall: Retailers
Details
Original published date: 2025-04-11
Alert / recall type
Health product recall
Category
Health products – Drugs
Companies
Nora Pharma Inc.
1565 boulevard Lionel-Boulet
Varennes, QC
J3X 1P7
Published by
Health Canada
Audience
General public
Healthcare
Industry
Distribution
National
Recall class
Type II
Identification number
RA-77266