Ottawa – Some time back Health Canada published amendments to the Hazardous Products Regulations (HPR) in the Canada Gazette, Part II, to align with the 7th revised edition, and certain provisions of the 8th revised edition, of the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). The amendments came into force on December 15, 2022. Canada Gazette, Part II, Volume 157, …
Trulicity now indicated as adjunct to diet, exercise
TORONTO – In recent past, Health Canada approved Trulicity® (dulaglutide) to reduce the risk of non-fatal stroke in adults with type 2 diabetes mellitus who have multiple cardiovascular risk factors or established cardiovascular disease, as an adjunct to diet, exercise, and standard of care therapy. This decision makes Eli Lilly and Company’s Trulicity the first and only GLP1 receptor agonist (RA) approved …
Protect yourself from bug bites
Ottawa – Health Canada always reminds Canadians to protect themselves from bug bites by safely using bug spray and other insect repellents. Bug bites can cause a number of health problems from itchiness and irritation, to potentially serious diseases. Personal insect repellents can help protect you from mosquito, blackfly and tick bites, but it’s important to remember that they should …
Lilly’s tirzepatide is superior to Wegovy in head-to-head trial
TORONTO– Eli Lilly Canada today announced topline results from the SURMOUNT-5 Phase 3b open-label randomized clinical trial. Tirzepatide provided a 47% greater relative weight loss compared to Wegovy® (semaglutide). On average, tirzepatide led to a superior weight loss of 20.2% compared to 13.7% with Wegovy. At 72 weeks, tirzepatide was superior to Wegovy on both the primary endpoint and all five …
Health Canada releases new data on cannabis use in Canada
Ottawa – An important part of the Government of Canada’s public health approach to legalizing and strictly regulating cannabis is ongoing and comprehensive surveillance, data collection and evidence gathering. The results from the annual Canadian Cannabis Survey provide a snapshot of public health and public safety data on cannabis in Canada. Health Canda published the 2024 Canadian Cannabis Survey (CCS) results. …
Feds announce investment in Québec-based Aramis Biotechnologies
Québec – The Government of Canada is investing in a robust, dynamic and resilient life sciences ecosystem capable of responding to current and future health emergencies. Since March 2020, over $2.2 billion has been invested to strengthen domestic biomanufacturing capabilities and help keep Canadians healthy and safe. Jean-Yves Duclos, Minister of Public Services and Procurement and Quebec Lieutenant, on behalf of …
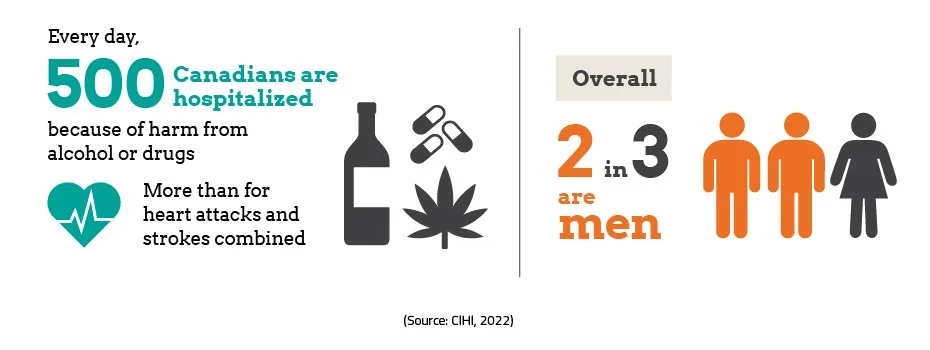
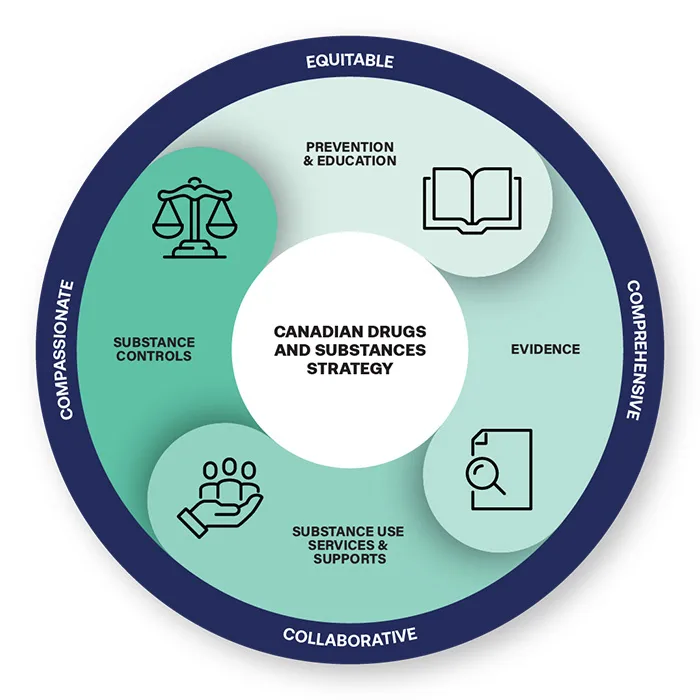
Improving health outcomes for those at risk of substance-related harm, overdose
St. Catharines – The overdose crisis is one of the most serious public health crises our country has ever faced. It is driven by a dangerous illegal synthetic drug supply that is unpredictable and increasingly toxic. Too many Canadians have lost their lives to this public health crisis. Canada’s approach is focused on providing access to a full continuum of …
Health Canada trying to reduce incidence of disease
Ottawa – One of Health Canada’s mandates is to reduce the incidence of disease and conditions among Canadians. Many science and health experts research and monitor diseases and you’ll find many of their reports and publications in this section. You’ll find information about many diseases and conditions, including their symptoms and treatments. Because healthy lifestyle choices reduce the risk of …
PMPRB report reviews new drugs, impact on future spending in Canada
Ottawa – The latest edition of Meds Pipeline Monitor, from the Patented Medicine Prices Review Board, provides stakeholders with a list of new medicines in the late stages of clinical evaluation that may significantly impact clinical practice or drug spending in the coming years. There were over 12,000 drugs in the pipeline in 2023, increasing by an average of 19% per …
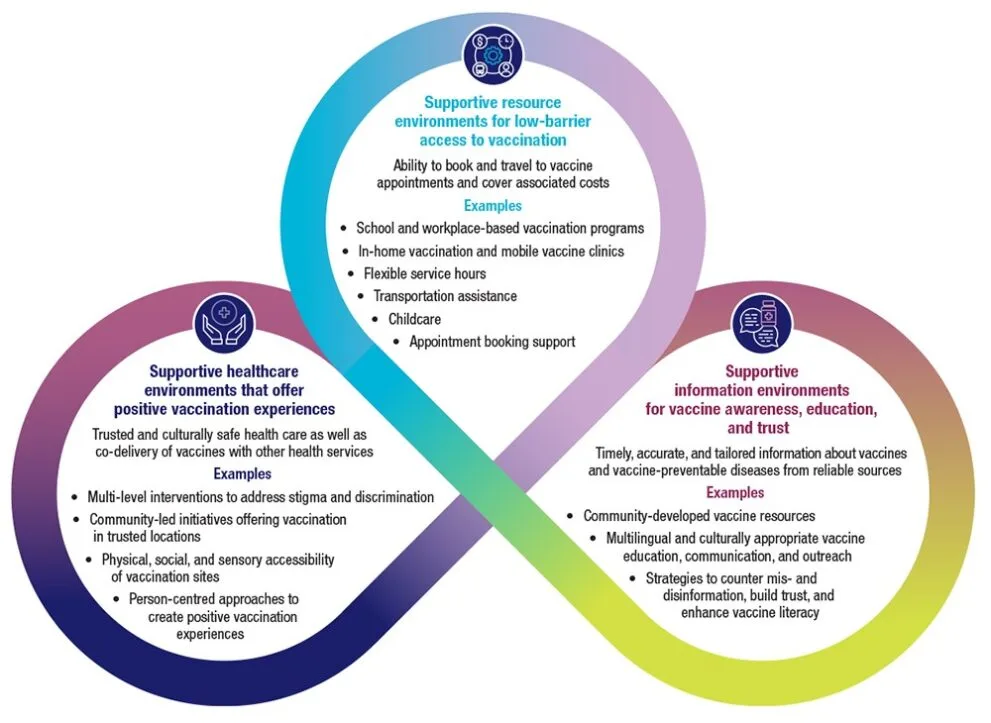
Supporting rapid responses to substance use, overdose crisis
Ottawa, ON | Health Canada No community has been left untouched by the toxic drug and overdose crisis. Its tragic impacts are felt among friends, families and neighbours. Too many Canadians have lost their lives to this public health crisis. Canada’s approach is focused on providing access to a full continuum of health care services and leveraging every tool at …